Prescription 2014: Exercise Your Liver
November 10, 2014 Written by JP [Font too small?]By now, the health benefits of exercise have been fairly well established in the scientific literature. In fact, there’s very little controversy or debate regarding the general value of physical activity on everything from cardiovascular to mental health. Even so, research into the therapeutic effects of exercise is ongoing and continues to reveal intriguing, new applications. One of the more exciting findings of late is the role which aerobic exercise plays in the promotion of liver health.
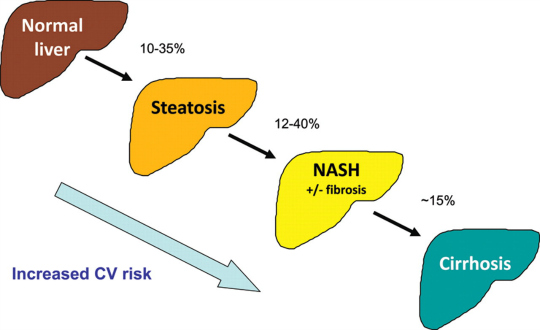
The liver is the primary organ that assists the body to process toxins. In addition, it aids the digestive and immune systems, filters blood, stores nutrients and much, much more. Excessive alcohol use, exposure to environmental chemicals, medications, obesity, poor diet and viral infections are established culprits that can damage and/or stress the liver. Herbal remedies and nutritional supplements are frequently recommended and used by consumers and practitioners alike in an attempt to protect the liver from such insults. And, in some instances, there is a scientific rationale for using certain herbal extracts, including cinnamon, milk thistle and synbiotics (i.e. pre- and probiotics). This is especially true for those living with a relatively common liver condition known as nonalcoholic fatty liver disease (NAFLD).
A review in the November 2014 issue of the European Journal of Applied Physiology reports that, “regular, moderate physical activity enhances liver health” in part by improving blood flow to the liver, lowering inflammation, supporting hepatic carbohydrate metabolism and, ultimately, lowering fat build up in this vital organ. In one study, walking on a treadmill for a total of 40 minutes (5 minute warm up, 30 minute walking, 5 minute cool down) three-times weekly was enough to reduce various inflammatory markers and elevated liver enzymes in patients with non-alcoholic steatohepatitis (NASH) – an inflammatory disease that causes liver scarring and can lead to cirrhosis. Other trials have concluded that non-competitive aerobic training is also an effective adjunct for those living with chronic hepatitis C. As a bonus, improvements in insulin sensitivity, oxidative capacity and psychological well-being were noted in much of the research. These findings provide adequate cause to recommend moderate aerobic exercise for virtually anyone who is interested in properly caring for their liver.
Note: Please check out the “Comments & Updates” section of this blog – at the bottom of the page. You can find the latest research about this topic there!
To learn more about the studies referenced in today’s column, please click on the following links:
Study 1 – Cinnamon May Have Therapeutic Benefits On Lipid Profile, Liver … (link)
Study 2 – Hepatoprotective Effect of Silymarin (Milk Thistle) … (link)
Study 3 – A Randomized, Controlled, Double-Blind, Pilot Study of Milk Thistle … (link)
Study 4 – Synbiotic Supplementation In Nonalcoholic Fatty Liver Disease: A … (link)
Study 5 – Effects of Physical Activity Upon the Liver … (link)
Study 6 – Markers of Liver Function & Inflammatory Cytokines Modulation By … (link)
Study 7 – Liver Enzymes and Psychological Well-Being Response to Aerobic … (link)
Study 8 – Effect of Exercise Training & Isoflavones On Hepatic Steatosis In … (link)
Study 9 – Short-Term Exercise Reduces Markers of Hepatocyte Apoptosis … (link)
Study 10 – Randomized Trial of Exercise Effect On Intrahepatic Triglyceride … (link)
The Link Between Fatty Liver and Cardiovascular Disease (CVD)
Source: Eur Heart J. 2012 May;33(10):1190-200. (link)
Tags: Inflammation, Liver, Milk Thistle
Posted in Alternative Therapies, Detoxification, Exercise


November 11th, 2014 at 5:56 pm
Hello JP
I am fighting dawn syndrome myself (liver dumping sugar) and have started on Himalaya Livercare now (LIV 52) and i am constantly looking for ways to improve my morning fasting numbers and this is all good information. Thank you again JP!
Best
Giri.
November 12th, 2014 at 1:02 pm
Hi, Giri.
I hope the combination of LIV 52 and exercise helps!
You’ve probably read the article below. But, if not, it offers additional suggestions.
http://www.mendosa.com/blog/?p=232
Be well!
JP
March 26th, 2015 at 7:53 pm
Update: Concerned about fatty liver? Consider a cup of coffee prior to exercise …
http://www.nature.com/ejcn/journal/vaop/ncurrent/full/ejcn201523a.html
Eur J Clin Nutr. 2015 Mar 25.
Coffee but not green tea consumption is associated with prevalence and severity of hepatic steatosis: the impact on leptin level.
BACKGROUND/OBJECTIVES: Most of the studies that have investigated the association between coffee consumption and hepatic steatosis have been experimental and small-scale clinical studies. As a result, epidemiological studies are scarce. To clear the association, we conducted a cross-sectional study and investigated the effects of coffee consumption with those of green tea consumption.
SUBJECTS/METHODS: We analyzed 1024 Japanese male workers. The diagnosis of hepatic steatosis was based on ultrasonography. We divided coffee and green tea consumption into the following three categories: non-drinker; 1-2 cups/day and ⩾3 cups/day. To investigate the association between hepatic steatosis and coffee or green tea consumption, we calculated the odds ratio (OR) and adjusted the means of leptin levels on each severity of hepatic steatosis.
RESULTS: A total of 265 of our subjects (25.9%) were diagnosed with hepatic steatosis. The ORs of the group of subjects who drank >3 cups of coffee/day was significantly lower compared with that of the noncoffee drinker group (OR 0.59, 95% confidence intervals 0.38-0.90, P=0.03). Although there was a significant difference between coffee consumption and leptin level only in the asymptomatic group, we found a decreasing trend in the asymptomatic and moderate-severe hepatic steatosis group. We did not find the same relationships in green tea consumption.
CONCLUSIONS: Although we did not find an association between hepatic steatosis and green tea consumption, coffee may have beneficial effects on hepatic steatosis. In addition, we produced one possible hypothesis that coffee consumption negatively associates with leptin levels in hepatic steatosis.
Be well!
JP
June 1st, 2015 at 2:07 pm
Update 06/01/15:
http://www.nmcd-journal.com/article/S0939-4753%2815%2900103-9/abstract
Nutr Metab Cardiovasc Dis. 2015 Apr 25.
A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease.
BACKGROUND AND AIMS: Very little information is available on whether docosahexaenoic acid (DHA) supplementation has a beneficial effect on liver fat and cardiovascular disease (CVD) risk factors in children with nonalcoholic fatty liver disease (NAFLD). In a double-blind, placebo-controlled randomized trial we investigated whether 6-month treatment with DHA improves hepatic fat and other fat depots, and their associated CVD risk factors in children with biopsy-proven NAFLD.
METHODS AND RESULTS: Of 58 randomized children, 51 (25 DHA, 26 placebo) completed the study. The main outcome was the change in hepatic fat fraction as estimated by magnetic resonance imaging. Secondary outcomes were changes in visceral adipose tissue (VAT), epicardial adipose tissue (EAT), and left ventricular (LV) function, as well as alanine aminotransferase (ALT), triglycerides, body mass index-standard deviation score (BMI-SDS), and insulin sensitivity. At 6 months, the liver fat was reduced by 53.4% (95% CI, 33.4-73.4) in the DHA group, as compared with 22.6% (6.2-39.0) in the placebo group (P = 0.040 for the comparison between the two groups). Likewise, in the DHA group VAT and EAT were reduced by 7.8% (0-18.3) and 14.2% (0-28.2%), as compared with 2.2% (0-8.1) and 1.7% (0-6.8%) in the placebo group, respectively (P = 0.01 for both comparisons). There were no significant between-group changes for LV function as well as BMI-SDS and ALT, while fasting insulin and triglycerides significantly decreased in the DHA-treated children (P = 0.028 and P = 0.041, respectively).
CONCLUSIONS: DHA supplementation decreases liver and visceral fat, and ameliorates metabolic abnormalities in children with NAFLD.
Be well!
JP
June 5th, 2015 at 11:55 pm
Update 06/05/15:
http://onlinelibrary.wiley.com/doi/10.1002/oby.21058/abstract
Obesity (Silver Spring). 2015 Jun;23(6):1259-66.
Hepatic steatosis is associated with lower levels of physical activity measured via accelerometry.
OBJECTIVE: Prior studies on the association of physical activity (PA) and nonalcoholic fatty liver disease are limited by reliance on subjective measures of PA. We examined the association between objectively measured PA and hepatic steatosis defined by computed tomography (CT).
METHODS: We conducted a cross-sectional study of 1,060 Framingham Heart Study participants who participated in the Multidetector CT 2 substudy and who underwent assessment of PA via accelerometry. Hepatic steatosis was estimated by liver attenuation, as measured by CT. We explored the relationship between liver attenuation and PA using multivariable regression models.
RESULTS: In multivariable-adjusted models, we observed an inverse association between PA and liver attenuation. Each 30 minutes/day increase in moderate to vigorous PA (MVPA) was associated with a reduced odds of hepatic steatosis (OR = 0.62, P < 0.001). This association was attenuated and no longer statistically significant after adjustment for body mass index (BMI) (OR = 0.77, P = 0.05) or visceral adipose tissue (VAT) (OR = 0.83, P = 0.18). Participants who met the national PA recommendations of engaging in ≥150 minutes/week of MVPA had the lowest odds of hepatic steatosis, even after adjusting for BMI (OR = 0.63, P = 0.007) or VAT (OR = 0.67, P = 0.03). CONCLUSIONS: There is an inverse association between PA and hepatic steatosis. Participants who met the national PA guidelines had the lowest prevalence of hepatic steatosis. Be well! JP
July 9th, 2015 at 2:14 pm
Update 07/09/15:
https://www.thieme-connect.com/DOI/DOI?10.1055/s-0035-1549853
Int J Sports Med. 2015 Jun 19.
Simple Resistance Exercise helps Patients with Non-alcoholic Fatty Liver Disease.
To date, only limited evidence has supported the notion that resistance exercise positively impacts non-alcoholic fatty liver disease. We evaluated the effects of resistance exercise on the metabolic parameters of non-alcoholic fatty liver disease (NAFLD) in 53 patients who were assigned to either a group that performed push-ups and squats 3 times weekly for 12 weeks (exercise group; n=31) or a group that did not (control; n=22). Patients in the control group proceeded with regular physical activities under a restricted diet throughout the study. The effects of the exercise were compared between the 2 groups after 12 weeks. Fat-free mass and muscle mass significantly increased, whereas hepatic steatosis grade, mean insulin and ferritin levels, and the homeostasis model assessment-estimated insulin resistance index were significantly decreased in the exercise group. Compliance with the resistance exercise program did not significantly correlate with patient background characteristics such as age, sex, BMI and metabolic complications. These findings show that resistance exercise comprising squats and push-ups helps to improve the characteristics of metabolic syndrome in patients with non-alcoholic fatty liver disease.
Be well!
JP
July 14th, 2015 at 3:13 pm
Update 07/14/15:
http://www.ncbi.nlm.nih.gov/pubmed/26153591
MMW Fortschr Med. 2014 Dec 15;156 Suppl 4:120-6.
[Effect of silymarin on liver health and quality of life. Results of a non-interventional study].
BACKGROUND: Many drugs are known to have hepatotoxic side effects. The effect of silymarin on liver function and liver-injury-impaired quality of life under daily practice conditions in patients with elevated values of liver enzymes was evaluated in the present non-interventional study.
METHOD: Patients with drug-induced elevated aminotransferase levels and indication for silymarin (Legalon forte) treatment for 2 to 3 months were documented prospectively over 4 months. At baseline, after 2 and 4 months, respectively, the following parameters were documented: alanine transaminase (ALT), aspartate transaminase (AST), γ-glutamyltransferase (GGT), alkaline phosphatase, total bilirubin, presence of liver-related skin symptoms and discoloured urine, severity of liver-related symptoms and quality of life.
RESULTS: In total, 190 patients (53.2% male, median age 60.0 years [range 19-81]) from 48 centres participated in the non-interventional study. Among potentially hepatotoxic drugs, analgesics/anti-inflammatory drugs were used most frequently (45.8%). These drugs have been administered for a median period of 2.8 years (range 0.0-26.1). At baseline, all patients had elevated levels of ALT, AST or GGT. Fatigue, flatulence, upper abdominal discomfort, lethargy, and joint complaints were the most severe liver-related symptoms and prevalent in over 62% of patients. Quality of life was affected in 88.7% of patients. Significant reductions were achieved in all documented laboratory parameters (p < 0.001), leading to marked improvement in liver-related symptoms and increased quality of life already after 2 months. The percentage of patients with liver enzymes in the normal range increased considerably within 4 months. No adverse drug reactions were observed. CONCLUSIONS: Silymarin is a safe and efficacious treatment option for patients with elevated liver enzymes. A benefit in terms of liver-related symptoms as well as quality of life and performance was demonstrated already after 2 months of treatment. Be well! JP
December 7th, 2015 at 7:01 pm
Updated 12/07/15:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4644631/
Hepat Mon. 2015 Oct 10;15(10):e31434.
Effect of Aerobic and Resistance Exercise Training on Liver Enzymes and Hepatic Fat in Iranian Men With Nonalcoholic Fatty Liver Disease.
BACKGROUND: Nonalcoholic fatty liver disease (NAFLD) has different prevalence rates in various parts of the world and is a risk factor for diabetes and cardiovascular disease that could progress to nonalcoholic steatohepatitis, cirrhosis, and liver failure.
OBJECTIVES: The current study aimed to investigate the effect of Aerobic Training (AT) and resistance training (RT) on hepatic fat content and liver enzyme levels in Iranian men.
PATIENTS AND METHODS: In a randomized clinical trial study, 30 men with clinically defined NAFLD were allocated into three groups (aerobic, resistance and control). An aerobic group program consisted of 45 minutes of aerobic exercise at 60% – 75% maximum heart rate intensity, a resistance group performed seven resistance exercises at intensity of 50% – 70% of 1 repetition maximum (1RM ) and the control group had no exercise training program during the study. Before and after training, anthropometry, insulin sensitivity, liver enzymes and hepatic fat were elevated.
RESULTS: After training, hepatic fat content was markedly reduced, to a similar extent, in both the aerobic and resistance exercise training groups (P ≤ 0.05). In the two exercise training groups, alanine amino transferase and aspartate amino transferase serum levels were significantly decreased compared to the control group (P = 0.002) and (P = 0.02), respectively. Moreover, body fat (%), fat mass (kg), homeostasis model assessment insulin resistance (HOMI-IR) were all improved in the AT and RT. These changes in the AT group were independent of weight loss.
CONCLUSIONS: This study demonstrated that RT and AT are equally effective in reducing hepatic fat content and liver enzyme levels among patients with NAFLD. However, aerobic exercise specifically improves NAFLD independent of any change in body weight.
Be well!
JP
January 29th, 2016 at 12:04 am
Updated 1/28/16:
http://www.nature.com/ijo/journal/vaop/naam/abs/ijo20164a.html
Int J Obes (Lond). 2016 Jan 20.
Strong and persistent effect on liver fat with a Paleolithic diet during a two-year intervention.
BACKGROUND/OBJECTIVES: Our objective was to investigate changes in liver fat and insulin sensitivity during a 2-year diet intervention. An ad libitum Paleolithic diet was compared to a conventional, low-fat diet.
SUBJECTS/METHODS: Seventy healthy, obese, postmenopausal women were randomized to either a Paleolithic diet or a conventional, low-fat diet. Diet intakes were ad libitum. Liver fat was measured with proton magnetic resonance spectroscopy. Insulin sensitivity was evaluated with oral glucose tolerance tests and calculated as HOMA-IR/Liver IR index for hepatic insulin sensitivity and OGIS/Matsuda for peripheral insulin sensitivity. All measurements were performed at 0, 6, and 24 months. 41 women completed the examinations for liver fat and were included.
RESULTS: Liver fat decreased after 6 months by 64% (95% CI: 54-74%) in the Paleolithic diet group and by 43% (27-59%) in the low-fat diet group (P<0.01 for difference between groups). After 24 months liver fat decreased 50% (25-75%) in the Paleolithic diet group and 49% (27-71%) in the low-fat diet group. Weight reduction between baseline and 6 months was correlated to liver fat improvement in the low-fat diet group (rs=0.66, P<0.01) but not in the Paleolithic diet group (rs=0.07, P=0.75). Hepatic insulin sensitivity improved during the first 6 months in the Paleolithic diet group (P<0.001 for Liver IR index and HOMA-IR), but deteriorated between 6 and 24 months without association to liver fat changes.
CONCLUSIONS: A Paleolithic diet with ad libitum intake had a significant and persistent effect on liver fat and differed significantly from a conventional low-fat diet at six months. This difference may be due to food quality, e.g. a higher content of mono- and polyunsaturated fatty acids in the Paleolithic diet. Changes in liver fat did not associate to alterations in insulin sensitivity.
Be well!
JP
February 27th, 2016 at 3:59 pm
Updated 02/27/16:
http://www.ncbi.nlm.nih.gov/pubmed/26916997?dopt=Abstract
Br J Nutr. 2016 Feb 26:1-8.
High serum carotenoids are associated with lower risk for developing elevated serum alanine aminotransferase among Japanese subjects: the Mikkabi cohort study.
Many recent studies have shown that antioxidant vitamins and/or carotenoids may reduce liver disease, but this association has not been well established with thorough longitudinal cohort studies. The objective of this study was to longitudinally investigate whether serum carotenoids at baseline are associated with the risk of developing elevated serum alanine aminotransferase (ALT) among Japanese subjects. We conducted a follow-up study of 1073 males and females aged between 30 and 79 years at baseline from the Mikkabi prospective cohort study. Those who participated in the baseline study and completed follow-up surveys were examined longitudinally. Exclusions included excessive alcohol consumption (≥60 g alcohol/d), hepatitis B and C and having a history of medication use for liver disease. A cohort of 213 males and 574 females free of elevated serum ALT (>30 IU/ml) at baseline was studied. Over a mean follow-up period of 7·4 (sd 3·1) years, thirty-one males and forty-nine females developed new elevated serum ALT. After adjustments for confounders, the hazard ratios for elevated serum ALT in the highest tertiles of basal serum β-carotene, β-cryptoxanthin and total provitamin A carotenoids against the lowest tertiles were 0·43 (95 % CI 0·22, 0·81), 0·51 (CI 0·27, 0·94) and 0·52 (CI 0·28, 0·97), respectively. For α-carotene and lycopene, borderline reduced risks were also observed; however, these were not significant. Our results further support the hypothesis that antioxidant carotenoids, especially provitamin A carotenoids, might help prevent earlier pathogenesis of non-alcoholic liver disease in Japanese subjects.
Be well!
JP
May 13th, 2016 at 10:29 pm
Updated 05/13/16:
http://www.cghjournal.org/article/S1542-3565%2816%2930149-5/abstract
Clin Gastroenterol Hepatol. 2016 May 4. pii: S1542-3565(16)30149-5.
Exercise-based Interventions for Non-alcoholic Fatty Liver Disease: a Meta-analysis and Meta-regression.
BACKGROUND & AIMS: The burden of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide. We performed a meta-analysis to determine the effectiveness of exercise-based lifestyle interventions on liver-specific endpoints in populations with NAFLD and underlying metabolic disorders such as obesity, type-2 diabetes, or the metabolic syndrome.
METHODS: We searched PUBMED-MEDLINE, EMBASE, and the Cochrane Central register, through 21st October, 2015 for randomized trials of exercise-based lifestyle interventions on endpoints such as intrahepatic lipid content and blood levels of alanine and aspartate aminotransferases. Effect sizes are reported as standardized mean difference and weighted mean difference values. To investigate heterogeneity, we performed sensitivity and meta-regression analyses. Results were reported according to the PRISMA statement.
RESULTS: We analyzed data from 28 trials. Physical activity, independently from diet change, was associated with a significant reduction in intra-hepatic lipid content (standardized mean difference, -0.69; 95% confidence interval [CI], -0.90 to -0.48) and with reductions in alanine aminotransferase (weighted mean difference, -3.30 IU/l; 95% CI, 5.57 to -1.04) and aspartate aminotransferase (weighted mean difference, -4.85 IU/l; 95% CI, -8.68 to -1.02). By meta-regression, we found individuals with increasing body mass index to be increasingly more likely to benefit from the intervention (beta coefficient = -0.10; P=.037). We recorded no effect modification by variables related to the intensity of the intervention.
CONCLUSIONS: In a meta-analysis of randomized trials, we found strong evidence that physical activity reduces intrahepatic lipid content and markers of hepatocellular injury in patients with NAFLD. This effect correlated with baseline body mass index.
Be well!
JP
June 28th, 2016 at 12:28 pm
Updated 06/28/16:
http://bjsm.bmj.com/content/early/2016/06/17/bjsports-2016-096197.long
Br J Sports Med. 2016 Jun 17.
Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis.
OBJECTIVE: Exercise training has been shown to have beneficial effects on liver function in adults overweight or with fatty liver disease. To establish which exercise programme characteristics were likely to elicit optimal improvements.
DESIGN: Systematic review and meta-analysis of randomised, controlled trials.
DATA SOURCES: PubMed, CINAHL and Cochrane controlled trials registry searched (1966 to 2 October 2015).
ELIGIBILITY CRITERIA FOR SELECTING STUDIES: Exercise intervention, with or without dietary intervention, versus usual care in adults undertaking, exercise training, who were overweight, obese or exhibited fatty liver disease (non-alcoholic fatty liver disease or non-alcoholic steatohepatitis).
RESULTS: We included 21 randomised controlled trials, totalling 1530 participants. Exercise intervention studies with total exercise programme workload >10 000 kcal produced significant improvements in intrahepatic fat, -3.46% (95% CI -5.20% to -1.73%), p<0.0001, I2=73%; effect size (standardised mean difference, SMD) -1.77 (-3.11 to -0.42), p=0.01, I2=77%. When data from only exercise studies were pooled, there was a reduction in fasting free fatty acids (FFAs) -74.15 µmol/L (95% CI -118.47 to -29.84), p=0.001, I2=67% with a large effect size (SMD) -0.94 (-1.36 to -0.52), p<0.0001, I2=0%. When data from only exercise studies were pooled, there was a significant reduction in insulin MD -1.88 UL (95% CI -3.43 to -0.34), p=0.02, I2=31%. The liver enzymes, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transpeptidase, were not significantly altered with exercise.
CONCLUSIONS: Exercise training reduces intrahepatic fat and FFAs while increasing cardiorespiratory fitness. An aggregate exercise programme energy expenditure (>10 000 kcal) may be required to promote reductions in intrahepatic fat.
Be well!
JP
September 9th, 2016 at 8:11 pm
Updated 09/09/16:
http://press.endocrine.org/doi/pdf/10.1210/jc.2016-2353
J Clin Endocrinol Metab. 2016 Sep 1:jc20162353.
Exercise training reduces liver fat and increases rates of VLDL clearance, but not VLDL production in NAFLD.
Context: Randomised controlled trials in non-alcoholic fatty liver disease (NAFLD) have shown that regular exercise, even without calorie restriction, reduces liver steatosis. A previous study has shown that 16 weeks supervised exercise training in NAFLD did not affect total VLDL kinetics.
OBJECTIVE: To determine the effect of exercise training on intrahepatocellular fat (IHCL) and the kinetics of large triglyceride-(TG)-rich VLDL1 and smaller denser VLDL2 which has a lower TG content. Design A 16 week randomised controlled trial. Patients 27 sedentary patients with NAFLD. Intervention Supervised exercise with moderate-intensity aerobic exercise or conventional lifestyle advice (control). Main outcome Very low density lipoprotein1 (VLDL1) and VLDL2-TG and apolipoproteinB (apoB) kinetics investigated using stable isotopes before and after the intervention.
RESULTS: In the exercise group VO2max increased by 31±6% (mean±SEM) and IHCL decreased from 19.6% (14.8, 30.0) to 8.9% (5.4, 17.3) (median (IQR)) with no significant change in VO2max or IHCL in the control group (change between groups p<0.001 and p=0.02, respectively). Exercise training increased VLDL1-TG and apoB fractional catabolic rates, a measure of clearance, (change between groups p=0.02 and p=0.01, respectively), and VLDL1-apoB production rate (change between groups p=0.006), with no change in VLDL1 -TG production rate. Plasma TG did not change in either group.
CONCLUSION: An increased clearance of VLDL1 may contribute to the significant decrease in liver fat following 16 weeks of exercise in NAFLD. A longer duration or higher intensity exercise interventions may be needed to lower plasma TG and VLDL production rate.
Be well!
JP
September 19th, 2016 at 4:22 pm
Updated 09/19/16:
http://www.journal-of-hepatology.eu/article/S0168-8278(16)30488-3/abstract
J Hepatol. 2016 Sep 14.
Aerobic versus Resistance Exercise in Non-alcoholic Fatty Liver Disease: A Systematic Review.
BACKGROUND & AIMS: Exercise is a first-line therapy for patients with non-alcoholic fatty liver disease (NAFLD). We sought to 1) summarize effective aerobic and resistance exercise protocols for NAFLD and 2) compare the effects and energy consumption of aerobic and resistance exercises.
METHODS: A literature search was performed using PubMed, Web of Science, and Scopas to January 28, 2016. From a total of 95 articles, 23 studies including 24 aerobic and 7 resistance exercise protocols were selected for the summary of exercise protocols. Twelve articles including 13 aerobic and 4 resistance exercise protocols were selected for the comparative analysis.
RESULTS: For aerobic exercise, the median effective protocol was 4.8 metabolic equivalents (METs) for 40 min/session, 3 times/week for 12 weeks. For resistance exercise, the median effective protocol was 3.5 METs for 45 min/session, 3 times/week for 12 weeks. Aerobic and resistance exercise improved hepatic steatosis. No significant difference was seen in the duration, frequency, or period of exercise between the two exercise groups; however,%VO2max and energy consumption were significantly lower in the resistance than in the aerobic group (50 [45-98] vs. 28 [28-28]%, P = 0.0034; 11,064 [6,394-21,087] vs. 6,470 [4,104-12,310] kcal/total period, P = 0.0475).
CONCLUSIONS: Resistance exercise improves NAFLD with less energy consumption. Thus, resistance exercise may be more feasible than aerobic exercise for NAFLD patients with poor cardiorespiratory fitness or for those who cannot tolerate or participate in aerobic exercise. These data may indicate a possible link between resistance exercise and lipid metabolism in the liver.
Be well!
JP
October 21st, 2016 at 12:58 pm
Updated 10/21/16:
https://www.ncbi.nlm.nih.gov/pubmed/27761987
Diabetes Obes Metab. 2016 Oct 19.
Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: a one-year follow-up study.
Exercise training can reduce hepatic fat accumulation and cardiovascular risk among patients with nonalcoholic fatty liver disease (NAFLD), but how long these benefits extend beyond the period of active intervention is unclear. Intrahepatic triglyceride (IHTG) content measured by proton magnetic resonance spectroscopy, and metabolic risk factors were assessed among 220 obese subjects with NAFLD who were randomly assigned to vigorous-moderate exercise, moderate exercise, or control over the subsequent 1 year after 12-month exercise intervention. IHTG content was significantly reduced in the two exercise groups compared to control over the 12-month active intervention. It remained significantly reduced by -2.39% in vigorous-moderate exercise compared to control at the 1-year follow-up (95% CI -4.72 to -0.05%, p = 0.045). Waist circumference and blood pressure remained significantly reduced in vigorous-moderate exercise and moderate exercise compared to control at the 1-year follow-up. Visceral adipose fat remained significantly reduced but with no differences among three groups. These findings suggest 12-month exercise intervention induced reductions in hepatic fat accumulation, abdominal obesity, and blood pressure for up to 1 year after the active intervention, with some attenuation of the benefits.
Be well!
JP
December 7th, 2016 at 12:11 am
Updated 12/06/16:
https://www.ncbi.nlm.nih.gov/pubmed/27917585
Liver Int. 2016 Dec 5.
The Preventive Effect of Sustained Physical Activity on Incident Nonalcoholic Fatty Liver Disease.
BACKGROUND & AIMS: Physical activity (PA) is inversely associated with nonalcoholic fatty liver disease (NAFLD) prevalence. However, few studies evaluated the effect of PA on NAFLD incidence in regard to visceral adipose tissue (VAT) and insulin resistance (IR). We investigated whether PA at baseline and change in PA during follow-up have any effect on incident NAFLD.
METHODS: We enrolled subjects who underwent health screenings between 2007 and 2008 and participated in voluntary follow-up between 2011 and 2013 (median 4.42 years). Incident NAFLD was defined as NAFLD absence at baseline and presence at follow-up by ultrasonography. PA was measured using a detailed questionnaire-based metabolic equivalent at baseline and follow-up; the difference during follow-up was calculated.
RESULTS: Of the 1,373 subjects enrolled, 288 (21.0%) developed NAFLD. Both total and leisure-time PA at baseline were inversely associated with incident NAFLD (p for trend=0.005 and 0.003, respectively). Decreased PA at follow-up was associated with increased incident NAFLD risk after adjusting for age, gender, body mass index, smoking, hypertension, diabetes, and diet [hazard ratio(HR) 1.45, 95% confidence interval (CI) 1.04-2.02, 4th (most decreased PA) vs. 1st quartile (increased PA), p=0.028]. This relationship was attenuated but remained statistically significant after adjustment for VAT(HR 1.48, 95% CI 1.06-2.06, 4th vs. 1st quartile) and IR(HR 1.59, 95% CI 1.11-2.27, 4th vs. 1st quartile).
CONCLUSIONS: This study shows an independent protective effect of PA at baseline on incident NAFLD after 4-year follow-up. Furthermore, sustained or increased PA had a preventive effect on incident NAFLD independent of VAT and IR.
Be well!
JP
April 27th, 2018 at 3:17 pm
Updated 04/27/18:
https://www.ncbi.nlm.nih.gov/pubmed/29696296
Diabetologia. 2018 Apr 26.
A heterogeneous response of liver and skeletal muscle fat to the combination of a Paleolithic diet and exercise in obese individuals with type 2 diabetes: a randomised controlled trial.
AIMS/HYPOTHESIS: The aim of the study was to investigate ectopic fat deposition and insulin sensitivity, in a parallel single-blinded randomised controlled trial, comparing Paleolithic diet alone with the combination of Paleolithic diet and exercise in individuals with type 2 diabetes.
METHODS: Thirty-two individuals with type 2 diabetes with BMI 25-40 kg/m2 and 30-70 years of age followed a Paleolithic diet ad libitum for 12 weeks. In addition, study participants were randomised by computer program to either supervised combined exercise training (PD-EX group) or standard care exercise recommendations (PD group). Staff performing examinations and assessing outcomes were blinded to group assignment. Thirteen participants were analysed in each group: hepatic and peripheral insulin sensitivity were measured using the hyperinsulinaemic-euglycaemic clamp technique combined with [6,6-2H2]glucose infusion, and liver fat was assessed by proton magnetic resonance spectroscopy; both analyses were secondary endpoints. Intramyocellular lipid (IMCL) content was measured by magnetic resonance spectroscopy as a secondary analysis. All examinations were performed at Umeå University Hospital, Umeå, Sweden.
RESULTS: Both study groups showed a median body weight loss of 7 kg. Fat mass decreased by 5.7 kg in the PD group and by 6.5 kg in the PD-EX group. Maximum oxygen uptake increased in the PD-EX group only. Liver fat showed a consistent reduction (74% decrease) in the PD group, while the response in the PD-EX group was heterogeneous (p < 0.05 for the difference between groups). IMCL content of the soleus muscle decreased by 40% in the PD group and by 22% in the PD-EX group (p < 0.05 for the difference between groups). Both groups improved their peripheral and adipose tissue insulin sensitivity, but not their hepatic insulin sensitivity. Plasma fetuin-A decreased by 11% in the PD group (p < 0.05) and remained unchanged in the PD-EX group. Liver fat changes during the intervention were correlated with changes in fetuin-A (rS = 0.63, p < 0.01). Participants did not report any important adverse events caused by the intervention. CONCLUSIONS/INTERPRETATION: A Paleolithic diet reduced liver fat and IMCL content, while there was a tissue-specific heterogeneous response to added exercise training. Be well! JP